The 90th anniversary of Fujifilm and Launch of Our New purpose of “Giving our World more Smiles.”
For the past nine decades, the FUJIFILM Group has consistently played an important role in improving healthcare all around the globe by connecting with people through their innovative products and services.
With a workforce of over 70,000 people around the globe, the company initially specialized in producing photographic film. Presently, FUJIFILM has undergone a major change and has emerged as a leader in multiple global industries such as healthcare, materials, business innovation, and imaging.
Beginning of FUJIFILM: Photographic Films, Motion-Picture Films and X-Ray Films
Established in 1934, Fuji Photo Film Co., Ltd. originally specialized in the production of photographic films. As time went on, the company expanded its product range to include motion-picture films and X-ray films. Eventually, Fuji Photo ventured into the market of optical glasses, lenses, and equipment.
In 1956, FUJIFILM was honored with the Deming Application Prize for its exceptional quality control in manufacturing, and it has remained committed to quality ever since.
Fuji Photo innovated in development of computed radiography (CR) which resulted in lower radiation exposure for technicians and patients. Further, the company's innovation extended to development of the SYNAPSE medical imaging, display materials, digital full-color copiers, production printers, and ATOMM technology used in magnetic tapes.
HealthCare as the Next Pillar of Growth: FUJIFILM HOLDINGS CORPORATION
The healthcare division of FUJIFILM fulfills medical needs, enhances care accessibility, and facilitates early disease detection with its advanced products and services.
By leveraging their extensive portfolio of Computed tomography (CT), Magnetic Resonance Imaging (MRI), X-ray diagnostic equipment, ultrasound, and endoscopy systems, as well as their advanced image processing, AI, and proprietary technologies, they are able to create new value in the medical diagnostic segment. Within the realm of advanced medical treatment, their Contract Development and Manufacturing Organization (CDMO) business is instrumental in ensuring a reliable availability of high-quality biopharmaceuticals and driving the progress of manufacturing gene and cell therapy drugs.
Making Healthcare More Safe: The Wako Acquisition
In a significant move in 2017, FUJIFILMS made a major decision to acquire Wako Pure Chemical Industries, marking a significant turning point for the company. Their achievement of this milestone resulted in an improved healthcare safety for the public through early detection and prevention of infections during treatment.
An infection can occur due to the presence of pyrogens in different elements, including drugs, medical equipment, water used in healthcare settings, and saline solutions. The important work done by Wako, now under the ownership of FUJIFILMS, actively contributes to preventing infections through their advancements in reagent-based testing methods for pyrogens.
Historical Significance of Pyrogen Testing
Kottmann first discussed the subject of pyrogenicity in 1906, which was later validated by Schaps in 1907. It was observed that when a man received a saline injection, he experienced an increase in body temperature. Through meticulous experiments, it was discovered that the fever resulted from the presence of impurities in injectables and other medical equipment.
To proactively address infections at their early stages, there was an emerging need to develop specialized testing techniques for medical equipment and medications. Due to the continuous progress in research and the rapid advancement in technology, a wide range of tests have been developed.
Fujifilm Wako and Pyrogen Testing
The in vivo rabbit pyrogen test, historically speaking, was one of the early techniques employed for pyrogen testing. Despite its previous usage, the traditional method has largely been substituted with the in vitro Limulus amebocyte lysate (LAL) assay, a test specifically designed to identify endotoxins. Moreover, in some scenarios, the monocyte activation test is employed to detect a broader range of pyrogens.
Furthermore, patients can develop peritonitis as a result of being exposed to dialyze products contaminated with peptidoglycan. FUJIFILMS Wako testing product portfolio has been expanded to include the detection of non-endotoxin pyrogens, specifically potential peptidoglycan contaminations, to contribute to a safer world for patients.
Modern Day Technologies At Wako
FUJIFILMS Wako chemicals have successfully developed an advanced product line that not only ensures precise and accurate results but also significantly simplifies the testing process for the operator. The development of various components is encompassed in this, such as reagents, accessories related to endotoxin tests, as well as the creation of endotoxin analysis systems like the Toxinometer ET-7000, the Toximaster® QC8 software, and other related tools.
FUJIFILM Wako Pure Chemical Corporation is a globally recognized supplier of high purity chemicals and reagents based in Japan. FUJIFILM Wako provides a line of reagents formulated to perform each of the three endotoxin detection assays: the Gel Clot Assay, the kinetic Turbidimetric Assay (KTA) and the Kinetic Chromogenic Assay (KCA).
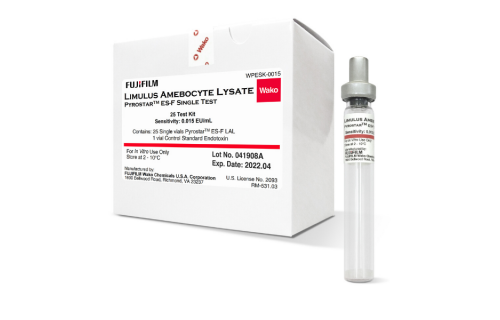
FUJIFILM Wako manufactures the PYROSTAR™ ES-F line of endotoxin testing reagents, which provide best-in-class sensitivity while ensuring no cross reactivity with other microbial contaminants.
Investing in a Better World
Endotoxines are classified as pyrogens, which are substances that cause fever, next to non-endotoxin pyrogens (NEPs) and are a crucial element of the outer membrane in gram-negative bacteria. If products contaminated with endotoxins are administered parenterally, the immune response they may elicit can be so severe that it can lead to fatality. The presence of endotoxins in the environment is widespread, making it essential to conduct testing for endotoxin contamination in injectable pharmaceuticals, medical devices, and their raw materials. This stringent testing protocol is necessary to uphold patient safety and well-being.
From this foundation, FUJIFILM established a new Group Purpose: “Giving our world more smiles.” The FUJIFILM Group remains committed to delivering innovative value and attaining sustainable growth. As a general reagent manufacturer, FUJIFILM Wako Pure Chemical Corporation has been delivering high-quality laboratory chemicals, specialty chemicals, and clinical diagnostic reagents to cater to different customer requirements.
FUJIFILM is dedicated to advancing science and is committed to developing cutting-edge products and technologies. We are actively working on creating high-quality reagents to support the scientific community.
In the journey of the past nine decades, FUJIFILM has evolved into a global, multi-national company innovating diverse businesses in healthcare, materials, business innovation, and imaging. The fresh purpose reflects the company’s dedication to bringing diverse ideas, unique capabilities, and extraordinary growth.